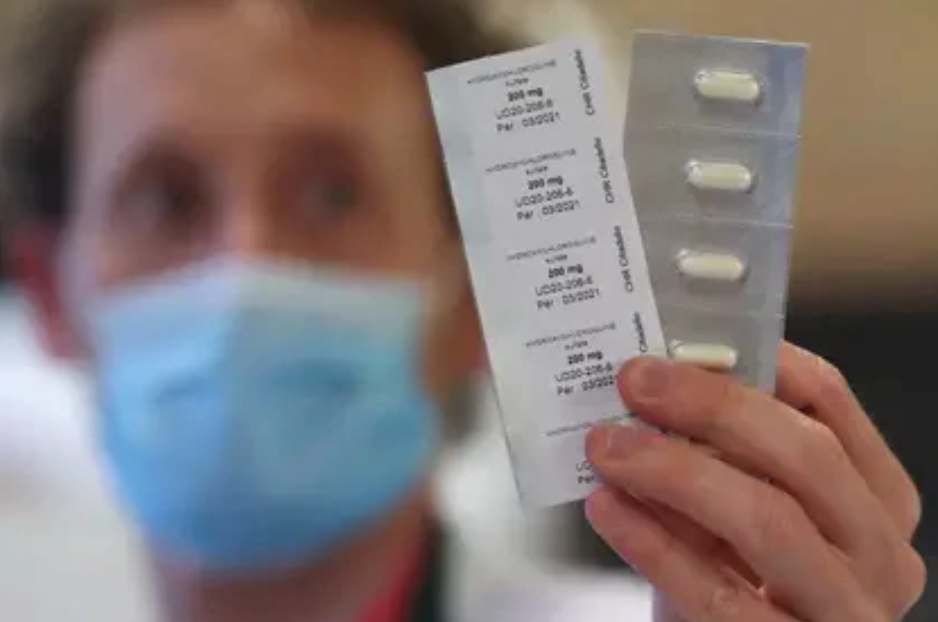
In its fight to contain the health crisis of the coronavirus in Mexico, the Ministry of Health will supply hydroxychloroquine to 20,000 outpatients of coronavirus starting next week.
It is a treatment to combat other autoimmune diseases, and the Federal Commission for the Protection against Health Risks (Cofepris) has already authorized the Ssa to administer the drug.
The drug will be offered to patients who have the virus, despite the fact that various scientists have warned that it increases the risk of arrhythmias and death and shows no benefit to COVID-19.